


The McCafferty Lab, based within the Department of Medicine at University of Cambridge, seeks to apply recombinant antibody technology towards the generation of therapeutic antibodies.
Over the last 30 years there has been a revolution in the ability of biotechnologists to create, engineer and express human antibodies resulting in approval of >200 therapeutic antibody drugs with over 1000 ongoing clinical trials. Working in both academic and commercial settings Affiliated Professor John McCafferty has been at the forefront of this revolution in antibody engineering.
John has founded 3 therapeutic antibody companies which have given rise to many antibodies in clinical trial as well multiple approved antibody drugs. He has invented a number of underpinning technologies in these companies including phage display, Mammalian Display and KnotBody® Technology.
The current lab within the Cambridge Institute for Therapeutic Immunology and Infectious disease at University of Cambridge seeks to apply these modern recombinant antibody technology towards the problems of the developing world with a particular focus on generation of human recombinant antivenoms.

The ability to engineer the affinity, specificity and developability properties of antibodies has accelerated development of therapeutics revolutionising treatment of diseases such as autoimmunity and cancer. The relatively high cost of antibody therapeutics however has limited their application to diseases of the developed world.
There is now an opportunity to extend these benefits into problems of the developing world. Advances in recombinant antibody technology over the last 30 years have reduced discovery costs, accelerated development timelines and de-risked programmes. Antibody production costs have fallen over the last few decades and initiatives such as the “Gates Foundation Grand Challenge” (in collaboration with LifeArc) seek to further drive down production costs to more affordable levels.
It is our vision that problems of the developing world such as snakebite envenomation and anti-microbial resistance will benefit from the advances in protein and peptide-based therapeutics enjoyed within the developed world.

Snakebite envenomation kills 100,000 people/year and causes debilitating injury to 100,000's more victims. The main therapeutic intervention for snakebite envenomation relies on the century-old approach of injecting victims with animal-derived polyclonal antiserum. While life-saving, the use of immune antiserum has a number of major limitations around consistency, redundancy and immunogenicity.
Recombinant antibody technology can resolve the complex immune repertoire arising from venom immunisation into its monoclonal components and allow harvesting of immune-derived high affinity antibodies. By isolating and expressing antibodies which neutralise individual toxic components, there is the potential to create defined, characterised cocktails of recombinant monoclonal antibodies.
Our collaborators at Institute Clodomiro Picado in Costa Rica produce EchiTab Plus, an equine anti-venom product in clinical use in sub-Saharan Africa. The McCafferty Lab have generated phage display libraries from the same immunised horses used to produce Echitab Plus. Individual venom components (e.g. phospholipases A2, snake venom metalloproteases) have been purified and hundreds of recombinant monoclonal antibodies have been isolated and characterised using a range of in vitro functional assays.
This project builds on our earlier studies targeting venom neurotoxins. This includes generation of broadly cross-reactive human antibodies neutralising cobra neurotoxins.
Team members pictured at the Oxford Venoms conference in 2024 with Andres Sanchez (collaborator at Institute Clodomiro Picado, Costa Rica), John McCafferty, Sandra Ergueta Carballo, Rhys Dunphy, Jonathon Rockman, Sheik Mohammed Arif.


John McCafferty has co-founded three companies in the recombinant antibody field, leading to significant commercial success and bringing multiple drugs to market.


Cambridge Antibody Technology (CAT), founded in 1990 by Dave Chiswell, Greg Winter and John McCafferty, was one of the big early successes in the UK Biotechnology sector. During their first year the company demonstrated display of functional antibodies on the surface of filamentous bacteriophage .

By constructing libraries of billions of clones, phage display technology unleashed the ability to generate fully human therapeutic antibodies. The technology proved to be robust and went on to widespread use in commercial and academic groups including the top 20 pharmaceutical companies world-wide.
CAT used the technology to develop the human antibody that become Adalimumab (Humira), which was the world’s top selling drug for many years and remains the highest selling drug on a cumulative basis (>$200 billion).

Cambridge Antibody Technology (CAT), founded in 1990 by Dave Chiswell, Greg Winter and John McCafferty, was one of the big early successes in the UK Biotechnology sector. During their first year the company demonstrated display of functional antibodies on the surface of filamentous bacteriophage .
Jim Marks, Tim Clackson, Dave Chiswell, Pete Jones, Andrew Griffiths, Jennie Hoogenboom, John McCafferty


John McCafferty and the team on the early stages of the lab consolidation


In 2012 McCafferty founded IONTAS, an innovative drug discovery company using recombinant antibody technology to generate human antibody drug candidates. The company initiated activities within the Biochemistry Department at University of Cambridge before moving to the Babraham Institute. IONTAS was founded without external investment and was profitable from the start with much of the revenue invested in developing innovative technologies.

IONTAS capitalised on the deep knowledge and experience of the founders in recombinant antibody technology and phage display, Working on over 40 projects for 20 different organisations the company achieving a 100% record of success in delivering on project goals. IONTAS operated a “hybrid model” and used the proceeds from the profitable service business to invest in a number of technology areas including Mammalian Display and KnotBody technology

Working with collaborators IONTAS completed many discovery programmes addressing unmet medical needs with at least 6 antibodies progressing to clinical trial.
IONTAS invented and patented a proprietary, Mammalian Display platform allowing selection not just on the basis of binding specificity but also based on the developability properties of the emerging antibodies, leading to faster development cycles.
The McCafferty group also invented and patented KnotBody technology which enables development of ion channel modulating antibodies. This technology has been brough to fruition through the foundation of Maxion Therapeutics where the technology has led to development of clinical candidates. IONTAS was sold in May 2020 and became part of the Fairjourney Biologics group, financed by GHO Capital (London).
mammalian display

part of the iontas team


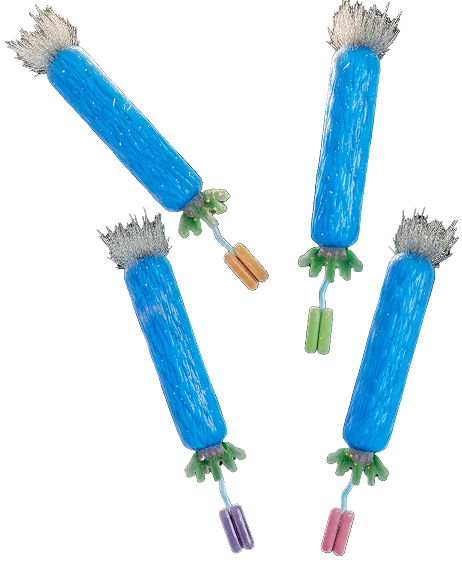
Ion channels are an important target class for which there are currently no antibody drugs. Dr McCafferty and colleagues at IONTAS invented a novel antibody fusion technology (KnotBody® Technology) which enables generation of ion channel-modulating antibodies, a class of target previously considered intractable to antibody development.

In order to overcome the challenge the group replace one of the surface loops of an antibody with naturally occurring, small peptides with ion channel modulating activity (“knottins”). The resultant fusion molecule (KnotBody® ) enjoys the best of both worlds combining the ion channel blockade of the knottin with the benefits of an antibody (i.e. long half-life, standard manufacturing and potential for engineering improved specificity and potency using phage display and mammalian display technologies).
Full realisation of the potential of this approach has necessitated development of parallel technologies for ion channel expression/purification as well as incorporation of high throughput ion channel functional assays within the company.

John founded and acted as initial CEO of Maxion Therapeutics to bring this technology to maturation and to steer KnotBody® molecules towards the clinic. Maxion has raised, over 2 rounds, a total of £70 million to develop the technology and advance its lead programme to the clinic led by new CEO Arndt Schottelius.


part of the maxion therapeutics team in the lab

John McCafferty has contributed key technologies that fundamentally advanced how therapeutic antibodies are discovered and engineered. From display platforms capable of exploring vast immune repertoires to innovative fusion formats inspired by natural ion-modulating peptides, these tools broadened what is scientifically and therapeutically possible. Together, they continue to influence modern antibody development across academia and industry.
A foundational method recognised by the 2018 Nobel Prize in Chemistry

Fusing natural ion-channel modulators with antibody frameworks
High-throughput antibody selection using engineered mammalian cells
Despite much effort by biotechnologists over the last 3 decades, creation of ion channel modulators has proven difficult. In contrast, nature produces small, cysteine-rich peptides, often as components of venoms, which have ion channel-modulating activity (“knottins”). Although knottins have the desired functional activity they are not optimal drugs.
Taking advantage of this Dr McCafferty and colleagues at Maxion Therapeutics (www.maxiontherapeutics.com) have developed KnotBody® Technology. With KnotBody® Technology the surface loop of an antibody is replaced by these naturally occurring “knottins” resulting in a fusion molecule which combines the best of both worlds. KnotBody® molecules enjoy the ion channel modulating activity of the knottin with the benefits of an antibody i.e. long half-life and standard manufacturing.
KnotBody® Technology works with phage and mammalian display and Maxion have used this to improve the affinity, specificity and biophysical properties of their lead molecules.
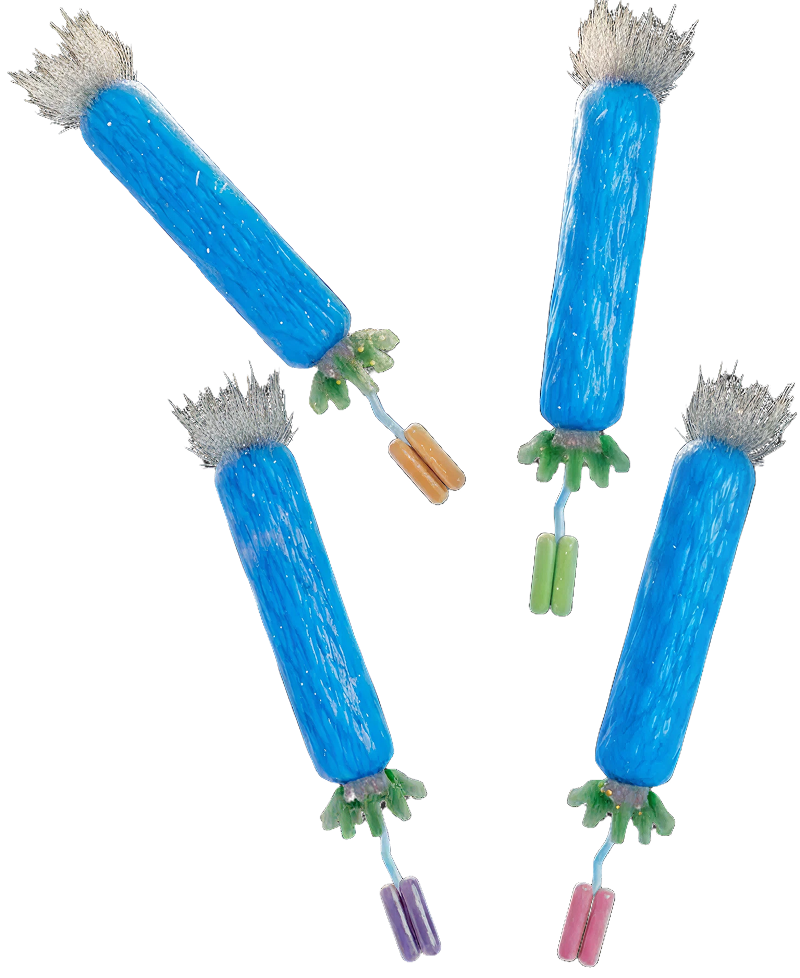
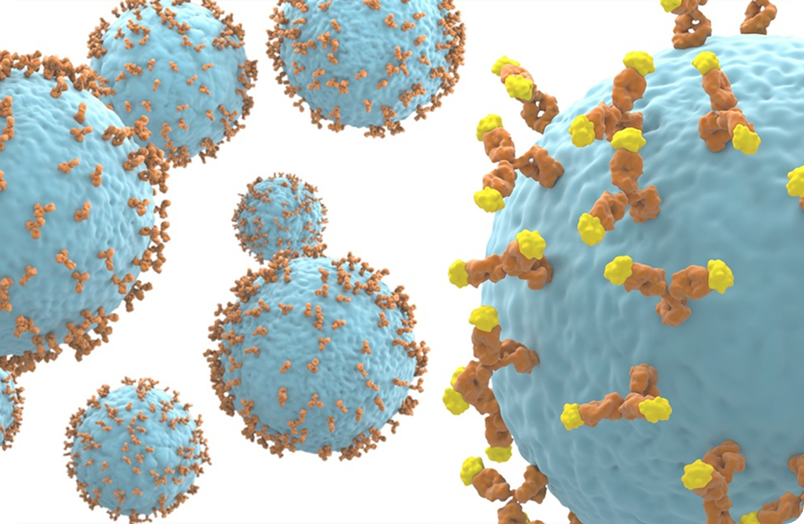
Antibody phage display operates by cloning antibody genes into the genome of the filamentous bacteriophage of E. coli in such a way that the encoded antibody is displayed on the phage surface while the encoding gene is on the inside. In this way the antibody gene can be selected on the basis of it’s encoded binding activity [McCafferty et al, 1990]. This breakthrough was quickly followed by the demonstration that “libraries” of antibody genes from immune or non-immune sources could be prepared. By constructing phage display libraries of billions of members it is possible via a “bio-panning” process to select antibodies to a wide range of target antigens. [Schofield et al, 2007]. The system is based on an in vitro approach which permits a high degree of control over selection conditions and antigen formats [Bradbury et al, 2015].
The underlying concept of linking genotype and binding phenotype has been extended to other systems including ribosome display, yeast display and mammalian display. Liberated from the need for immunisation these powerful technologies have also allowed the development of various alternative non- antibody scaffolds. The importance of the technology was recognised by the awarding of the 2018 Nobel prize for Chemistry to co-inventor Sir Gregory Winter.
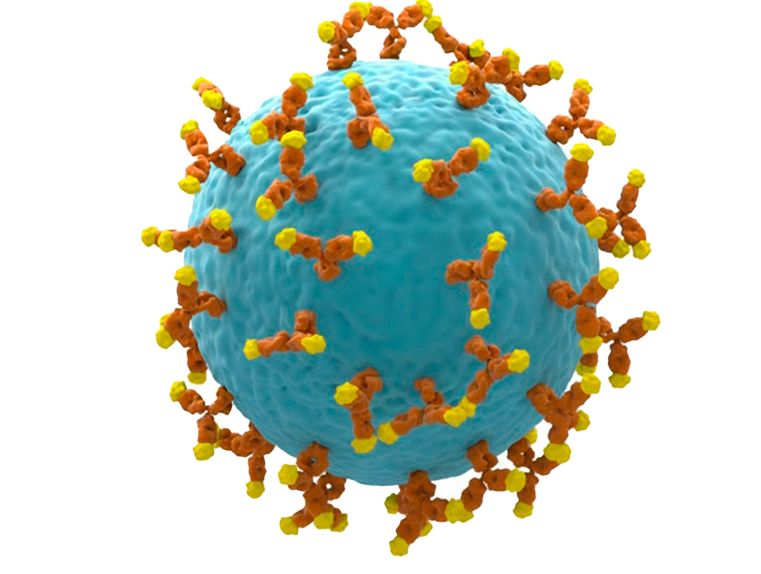
Mammalian display is based on the same principal as phage display-namely the creation of a ‘package” linking a binding molecule and its encoding gene. A particular problem with mammalian cells is the ability to create sufficiently large libraries while retaining a single antibody gene per cell. The McCaffery group lab at IONTAS solved this problem by integrating antibody genes into a single genomic locus using TALE nucleases or CRISPR/Cas9. This allows construction of massive collections of mammalian cell clones (10 7 -10810-100 million members) wherein each cell encodes and expresses an individual IgG- formatted antibody on the cell surface. This allowed direct screening by flow cytometry of millions of different antibodies expressed on the surface of mammalian cells (Parthiban et al, 2019).
This approach also allowed isolation not just based on binding affinity or specificity but also based on the biophysical properties of the encoded antibody allowing generation of high affinity, highly developable antibody drugs (Dyson et al 2020, Slavny et al 2024).